作者简介:: LIN Jia, female, Minhou, Fujian; Doctor, Senior Engineer, focusing on research and detection of trace evidence. E-mail: 12645923@qq.com
目的 建立固相支撑液液萃取结合液相色谱-质谱(SLE-LC-MS)检验全血中氟阿普唑仑和2-氯地西泮的方法。方法 经固相支撑液液萃取(经测试,其优于液液或固相萃取法)柱提取净化,应用电喷雾离子源(ESI)、多反应监测(MRM)正离子模式质谱进行定性及定量检验血样中氟阿普唑仑和2-氯地西泮。通过优化固相支撑液液萃取条件、色谱和质谱条件,采用液相色谱-质谱定性定量分析全血中氟阿普唑仑和2-氯地西泮。结果 三种方法(液液萃取法、固相萃取法、固相支撑液液萃取法)萃取全血中氟阿普唑仑和2-氯地西泮,结果显示固相支撑液液萃取法的回收率最高。在5~500ng/mL范围内全血中氟阿普唑仑和2-氯地西泮与峰面积呈现良好的线性关系( R2=0.999),氟阿普唑仑和2-氯地西泮的最低检出限分别为0.05ng/mL和0.1ng/mL,平均基质效应分别为77.5%和72.5%,平均回收率分别为95%和79%,方法处理效率分别为73%和72%。结论 该实验方法操作简单,结果准确,能满足全血中氟阿普唑仑和2-氯地西泮的检验需求。
Objective To establish a method for analyzing flualprazolam and 2-chlorodiazepam in whole blood with supported liquid-liquid extraction-high performance liquid chromatography/mass spectrometry (SLE-HPLC-MS).Methods The whole blood was extracted with the cartridge of supported liquid-liquid extraction (SLE) that is tested of excelling both liquid-liquid extraction (LLE) and solid-phase extraction (SPE), having its contained flualprazolam and 2-chlorodiazepam determined with the HPLC-MS system being selected of an ESI+ mode and multiple reaction monitoring (MRM).Results Through comparison of three extraction methods (SLE, LLE and SPE) for their efficiencies to analyze flualprazolam and 2-chlorodiazepam in whole blood by LC-MS, SLE was of the best extraction choice. Accordingly, Flualprazolam and 2-chlorodiazepam had rendered their linear calibration curves in the range of 5-500ng/mL, with their correlation coefficients ( R2) greater than 0.999, and the LODs being 0.05ng/mL and 0.1ng/mL, respectively. Besides, flualprazolam showed its mean matrix effect was 77.5%, mean recovery 95%, and average processing efficiency 73%, while 2-chlorodiazepam presenting its own respective parameters indicated above as 72.5%, 79%, and 72%. --Conclusion-- The method presented here is simple, rapid, sensitive and accurate for the determination of flualprazolam and 2-chlorodiazepam in whole blood samples.
Most methods for determination of the designer’ s benzodiazepines (Internet-available recreational drugs to which both flualprazolam and 2-chlorodiazepam belong) in biological fluids are based on immunoassays as they are rapid and easy to perform. Nevertheless, their major drawback is cross reactivity with relevant metabolites or other steroids, often leading to overestimation of steroid concentration [1, 2, 3]. Alternatively, LC-MS methods have been developed of detecting designer’ s benzodiazepines in biological matrices with eligible sensitivity and specificity for routine forensic toxicological analysis [4, 5, 6]. In addition, they allow the direct analysis of aqueous solutions, showing better suited for non-volatile compounds. Therefore, they are predominantly used to identify and quantify synthetic designer’ s benzodiazepines.
Several methods have been reported with different techniques for sample preparation such as the simple liquid-liquid extraction (LLE) that is easy to perform but requires a higher sample volume than the automation-enabled solid phase extraction (SPE) [7, 8]. Supported liquid extraction (SLE) has been applied in various fields, e.g., environmental analysis and food production [9, 10], but only recently has it begun to find a place in the field of toxicology [11]. It relies on an aqueous phase containing the analyte adsorbed onto a porous inert surface (e.g., diatomaceous earth) in a way that maximizes the surface area to absorb liquid. An immiscible solvent is followed to add for extracting the analyte from the aqueous phase. SLE purification is as both simple and fast as LLE and less time-consuming than SPE determination [12].
Flualprazolam and 2-chlorodiazepam are shown of their structures in Fig.1. They are the products only available in chemical laboratories and have never been officially and normally sold as pharmaceuticals. Yet, with allurement into their sedative and hypnotic effects, flualprazolam and 2-chlorodiazepam are readily adopted by criminals to engage into illegal acts. However, the poorly documented detection of the two drugs in biological samples poses a challenge to forensic toxicological laboratories.
In this paper, a simple HPLC-MS/MS method was described with only one-step extraction by SLE and water-based calibrators for simultaneous determination of flualprazolam and 2-chlorodiazepam. Results of full validation demonstrated that the method possesses favorable features in items of perfect selectivity and specificity, high detection sensitivity, improved peak shapes, reproducible retention time and high throughput for sample. The proposed method provides an appealing alternative for forensic toxicology as well as for therapeutic drug monitoring in hospital.
Flualprazolam was purchased from Cayman (Michigan, USA); 2-chlorodiazepam and diazepam-d5 (used as internal standard, IS) were from Sigma (MO, USA), all of HPLC-pure grade. Acetonitrile and methanol, products of Fisher Scientific (Fairlawn, NJ, USA). Formic acid, from Sigma-Aldrich (USA). ISOLUTE SLE+ 1 mL supported liquid extraction column, from Biotage (Charlotte, North Carolina, USA). Deionized water was generated with an Elix water purification system (Millipore, Molscheim, France). All glass vials and vial inserts were silanized and the screw caps were PTFE-lined, with all of them purchased from VWR International (Mississauga, Ontario, Canada).
Analyses were performed on an Agilent 1290 HPLC system with the SCIEX QTRAP 6500 MS/MS as the detector. Chromatographic data were recorded and analyzed by Analyst® software.
Separations were carried out on a Kinetex C18 column (3.0 mm× 100 mm, 2.6 μ m; PHENOMENEX, USA) that was maintained at the temperature of 35 ° C and run under a constant flow rate of 0.3 mL/min. The mobile phase was composed of 5 mmol/L ammonium formate in purified water (A) and acetonitrile (B), with both of them containing 0.1% formic acid. The selected gradient program was shown in Table 1. The sample-injecting volume was 1 μ L.
| Table 1 Gradient program for HPLC |
The quantitative analysis of flualprazolam and 2-chlorodiazepam was performed in MRM mode. Positive ionization mode (ESI+) was used for all analytes. The MS parameters were: curtain gas, 35 psi; ionspray voltage,
5 500 V; temperature, 550 ° C; ion source gas1, 55 psi; ion source gas2, 55 psi; collision gas, 10 psi. Each analyte was identified via two characteristic MRM transitions as shown in Table 2.
| Table 2 Mass spectrometric parameters for analytes and internal standard (IS) |
For blank samples, human venous whole bloods were obtained from a Fujian provincial hospital (Fuzhou, Fujian, China). These samples were screened before used as the negative controls, the blanks with internal standard, the spiked calibrators and the quality control samples. Authentic forensic samples of whole blood were preserved with sodium fluoride (Merck, Darmstadt, Germany) and potassium oxalate (BD, Plymouth, UK).
2.5.1 SLE
ISOLUTE SLE+ 1mL columns were used for blood sample preparation. 500 μ L sample of spiked blood (added of 1 ng/mL diazepam-d5, the IS) was transferred into an SLE+ column. A minimum positive pressure from an in-house system (0.1 bar) was applied to facilitate the sample to absorb into the cartridge in less than 10 s. After the analytes were allowed to equilibrate with the sorbent for a minimum of 5 min, the compounds were eluted with 1mL × 2 of ethyl acetate. The eluate was evaporated to dry under nitrogen at 45° C. The dried samples and blank samples were reconstituted with 400 μ L of acetonitrile/water (25/75, v/v) and standard solution of analytes in acetonitrile/water (25/75, v/v), respectively, and vortexed to mix for 2 min. The resulting solution was directly subject to LC-MS/MS analysis.
2.5.2 LLE
500 μ L sample of spiked blood (containing 1 ng/mL diazepam-d5, IS) was prepared through a liquid-liquid extraction with ethyl acetate/heptane mixture (4∶ 1, v/v) after the addition of 0.1 mol/L borate buffer (pH 9) and followed to be shaken for 5 min. Subjected to centrifugation at 3 000 r/min for 10 min, the extracted solution of spiked blood sample had its upper layer transferred into another test tube. With the above extraction step being repeated for three times with the 500 μ L spiked blood sample, the combined extract was evaporated to dry at 45 ° C under nitrogen gas. The dried sample was reconstituted with 400 μ L of acetonitrile/water (25/75, v/v) and subjected to LC-MS/MS analysis.
2.5.3 SPE
The blood samples were diluted with 3 mL of 0.1 mol/L phosphate buffer (pH 7.2), vortexed for 15 s, and then sonicated for 15 min. Resulted from centrifugation at 3 000 r/min for 5 min, the supernatant was loaded onto the HLB SPE cartridge preconditioned with 2 mL of at-first methanol and then distilled water. After loaded, the cartridge was washed with 2 mL of 5% methanol and the analytes were subsequently eluted with 3 mL of 20% methanol in water. Subsequently, the eluates were evaporated to dry under nitrogen at 45 ° C. The dried sample was reconstituted with 400 μ L of acetonitrile/water (25/75, v/v) and subjected to LC-MS/MS analysis.
The specificity and selectivity were tested with separate samples from two different human blood samples spiked. Fig.2 showed the total ion chromatograms (TIC) of flualprazolam, 2-chlorodiazepam and diazepam-d5 through LC-MS/MS, with them being eluted out at 7.70, 9.02 and 8.87 min, respectively. No peak was detected in the blank blood sample, and there was no interfering peaks derived from endogenous substances at the elution time of the analytes.
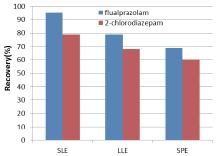
The extraction methods of LLE, SPE and SLE were compared. As shown in Fig.3, the extraction efficiencies for flualprazolam and 2-chlorodiazepam were improved most significantly with SLE. Therefore, SLE was selected to treat the sample.
The results of the matrix effect, recovery and processing efficiency of flualprazolam and 2-chlorodiazepam in human whole blood were summarized in Table 3. The mean matrix effect was 77.5% for flualprazolam and 72.5% for 2-chlorodiazepam. The mean recovery was 95% for flualprazolam and 79% for 2-chlorodiazepam. The mean processing efficiencies of flualprazolam and 2-chlorodiazepam were 73% and 72%, respectively.
| Table 3 The matrix effect, recovery and processing efficiency of flualprazolam and 2-chlorodiazepam |
The calibration curves showed good linearity over the concentrations ranging among 5.0-500 ng/mL, with correlation coefficient (R2) greater than 0.99. The mean slopes and intercept values of calibration curves for the flualprazolam and 2-chlorodiazepam were given in Table 4. The LOD values of flualprazolam and 2-chlorodiazepam were 0.05 ng/mL and 0.1 ng/mL, respectively. The low limit of quantification was 5 ng/mL for the two analytes, thus being defined as the lowest concentration in the calibration curve with acceptable precision and accuracy (± 20% bias).
| Table 4 Linearity and LODs for flualprazolam and 2-chlorodiazepam |
The intra- and inter-day precision and accuracy were summarized in Table 5. Precision (RSD) was shown as the coefficient of variation (CV%) of the concentrations determined by replicates of the QC samples. The supported liquid-liquid extraction method here-developed showed good precision and accuracy. From both the intra- and inter-day analyses, the accuracy ranged from 86.0% to 109.0% at low, medium or high concentrations, with the precision (RSD) values of CV% less than 6.2%.
| Table 5 Precision and accuracy for flualprazolam and 2-diclazepam in human whole blood spiked |
The established method was applied into several real forensic cases. Bloods from two sexually assaulted victims were collected for toxicological analysis. Flualprazolam and 2-chlorodiazepam were successfully detected in all samples, with their concentrations shown in Table 6.
| Table 6 Details of forensic cases and detection results from the established method |
In this study, an analytical method using SLE and liquid chromatography-tandem mass spectrometry was developed and validated for the determination and quantification of flualprazolam and 2-chlorodiazepam in human whole blood samples. The method was validated through the items of selectivity, sensitivity, matrix effect, recovery, processing efficiency, LOD, LLOQ, linearity, accuracy and precision, with all the parameters in accordance with the acceptable criteria. The validated method was successfully applied to determine the concentration levels of flualprazolam and 2-chlorodiazepam in whole bloods of victims from real cases.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|