社会快速变化导致的压力和/或诱惑,使得药物中毒正愈发显著地逐年增多,因而成为急救医学的一个突出方面。故建立一个快速准确的筛选方法从而鉴定中毒物质就显得尤为必需。但在韩国急救室环境下这样的一种系统性毒物毒理学研究仍不充分。因此,本研究旨在通过使用GC-MS仪器系统建立一个快速准确的方法以鉴定所涉药物,并对药物浓度与临床症状之间的关系作评估,从而为病人的恰当治疗提供借鉴和依据。为此,我们收集了2015年2至7月入住忠南国立大学医院的80位病人的血样,并以GC-MS进行分析。结果发现,唑吡坦是检出率最高的药物,共有15例,病人中唑吡坦的浓度在19.63~3605.85ng/mL之间,病人年龄从20到88岁。其中有5例(33%)企图自杀的病人仅服用了唑吡坦,另外10例则还选择了其他药品(一种或几种)配合使用。这些药品中,酒精3例;但曲马多被选配得最多,有4例;接下来是醋氨酚(亦即对乙酰氨基酚)3例;曲唑酮和氯苯吡胺各2例。另外,阿普唑仑、氯氮平、苯海拉明、喹硫平分别检出1次。80岁以上年龄病人组的唑吡坦浓度最高,但这与格拉斯哥昏迷评分、心理变化、服药剂量以及药物中毒后已过时间等因素却没有关联性。这很可能与服药量和/或同其他药品一起使用的准确信息缺乏有关。应可推断,所观察到的个体表现差异更多地与病人的代谢状况而不是服药剂量具有相关性。全部15例病人包括唑吡坦浓度最高者都在几天之内大为好转并出院。故唑吡坦用药过量的症状是中度的,并不会造成进一步的伤害。本研究对于完善分析体系从急救室病人样品中检测毒品能起到补充作用,对指导合理用药剂量有借鉴意义,对毒代动力学研究建立一套快速恰当的治疗程序具有帮助价值。此外,本文相关信息和结论对于法医毒理学确定与检测涉及唑吡坦中毒的死亡也有助益。
First Author: MOON Hantae (1989-), male, Korean, MS, Researcher in analysis of drugs. munankiki@naver.com
*Corresponding Author: CHUNG Heesun (1955-), female, Korean, Ph.D, Professor in analysis of drugs. hschung@cnu.ac.krIn accordance with the tension and/or temptation from rapid changes in society, intoxication is significantly increasing year by year and becoming a remarkable presence in emergency medicine. It is necessary to establish a fast and accurate screening method to identify the toxicants, but research for such systematic toxicological analysis in emergency room setting is insufficient in Korea. Hence, the purpose of this study was to establish a fast and accurate method for identifying the drugs using GC-MS and to evaluate the correlation between the blood concentration of drugs and the clinical symptoms for proper treatment of patients. In order to set up the analytical method, blood specimens were collected from 80 patients who were admitted to Chungnam National University Hospital from February to July in 2015 and were analyzed by GC-MS. As a result, zolpidem was the most frequent drug, detected in 15 cases, showing its blood concentration ranging from 19.63 to 3605.85 ng/mL in the patients aging from 20 to 88 years old. 5 of the cases were patients who ingested zolpidem for suicide attempt (33%). Ingestion of zolpidem alone was in 5 cases and ingestion with other drugs in 10 cases. Alcohol was detected in 3 cases. The most common drug taken together was tramadol, detected in 4 cases, followed by acetaminophen in 3 cases, and trazodone and chlorpheniramine in 2 cases, respectively. Alprazolam, clozapine, diphenhydramine and quetiapine were each detected once. The zolpidem concentration was the highest among the patients in their 80s (older than 80 years), but there was no correlation with GCS score, mental change, the ingested amount or elapsed time after intoxication. This is most likely due to the lack of accurate information on the ingested dose and other substances. It is supposed that the difference observed in each individual is associated with metabolism rather than ingested dose. All of 15 patients including those with high zolpidem concentrations improved within a few days and were discharged. Symptoms of intoxication by zolpidem overdose were moderate, no further damage observed. This study will supplement the analytical system for detecting toxicants from samples of emergency room patients, information of correct dosage, and toxicokinetics exploration to establish a procedure for fast and appropriate treatment. Besides, it will provide useful information for determining intoxication deaths related to zolpidem in forensic toxicology.
Rapid social changes cause a number of intoxication cases increasing annually, leaving acute intoxication becoming an important aspect of emergency medicine. It is very important to identify toxic drugs and chemicals in patients through a fast and accurate screening method.
In this study, the screening method was established and applied to blood specimens collected from 80 patients who were admitted to Chungnam National University Hospital from February to July in 2015. Expectedly, zolpidem was the most commonly detected drug, being detected in 15 out of 80 cases.
Zolpidem is structurally classified as an imidazopyridine, also chemically known as N, N-dimethyl-2-(6-methyl-2-p-tolylimidazo[1, 2-α ]pyridin-3-yl)aceta-mide [1]. Currently, zolpidem is the most prescribed sedative-hypnotic for treatment. It is a non-benzodiazepine hypnotic agent which has been shown to be effective in inducing and maintaining sleep in adults [2, 3, 4, 5, 6].
Zolpidem has been marketed in Europe since 1987, and was approved by US Food and Drug Administration (FDA) in April 1992. It is the 15th most prescribed drug in the US [7] and as a result, a high number of acute intoxication has been reported about zolpidem.
Acute intoxication related to zolpidem is often the stories from autopsy cases. According to Jones [8], 357 cases of death related to zolpidem occurred from 2001 to 2010 in Sweden. Jonsson [9] found that in Sweden, 25 out of 3560 autopsy cases were death related to zolpidem alone. Also, Tominaga [10] reported that zolpidem was detected in 3 cases from 808 autopsy cases during 2010 to 2014 in Japan. Thus, it can be seen that zolpidem affects the cause of death alone or with other drugs.
In Korea, zolpidem was detected in 16 autopsy cases conducted by National Forensic Service from 2006 to 2008, and discovered to be mainly used for suicide attempt as well as the cause of death due to acute intoxication [11].
The quantitation of zolpidem in 15 cases here showed a huge discrepancy between samples. Indeed, three cases revealed extremely high concentrations of zolpidem, over 1600 ng/mL, a concentration of comatose-fatality according to Schulz [12]. However, three patients in these cases were in neither comatose-fatal nor serious condition when admitted and fully recovered in a couple of days.
Thus in this study, the relationship between blood concentrations and clinical symptoms was studied so as to explain how patients survived at such a high level of the drug. These are very observation-worthy non-fatal cases with extreme high zolpidem concentrations.
Case 1: An 80 year-old male was admitted to the hospital after he failed to wake up. His mental state was stupor at the time of admission and GCS (Glasgow Coma Scale) was 7. Intubation was operated. There were no other unusual findings on brain MRI. Decreased consciousness was suspected due to drugs on the EEG (electroencephalogram) test. After consciousness was recovered, his CK (Creatine Kinase) was 1047 on the blood test. He was transferred to the division of pulmonology because aspiration pneumonia and rhabdomyolysis were suspected. Sample was collected 25 hours after intoxication.
Case 8: An 81 year-old female, suffering with Parkinson’ s and Alzheimer’ s disease, was admitted after overdosing her prescription drug, zolpidem. Her mental state was alert and GCS was 14 at the time of admission. Chest X-ray suggested pneumonia, but no other unusual findings were observed. Sample was collected 13 hour after intoxication.
Case 9: An 81 year-old female, suffering from depression, was admitted to the hospital after attempting suicide by ingesting zolpidem and half a bottle of alcohol, and then self-inflicting her right wrist. Her mental state was drowsy and GCS score was 8 at the time of admission. There were no other unusual findings. She was discharged next day. Sample was collected 9 hours after intoxication.
Case 12: An 88 year-old female, afflicting of anxiety disorder, was admitted after ingesting a large dose of hypnotics with alcohol. Her mental state was semi-coma and GCS score was 3 at the time of admission. Intubation was carried out. There were no other unusual findings. Sample was collected 11 hours after intoxication.
Case 14: An 82 year-old male showing decreased consciousness was admitted to the hospital’ s emergency room. According to the family, ingestion of hypnotics was suspected but no presence of clear evidence. His GCS score was 9 and mental state was stupor at the time of admission. Pulse rate (PR) was 125, respiratory rate (RR) 40, with a fever of 39.7 ° C. Intubation and ventilator were applied. Lactic acid was 4.7, WBC (White blood cell) 11 800, and CRP (C-reactive protein) 1.2, according to the blood test. Aspiration pneumonia was suspected based on chest X-ray and CT scan. He was transferred to a care facility as DNR (Do Not Resuscitate) state. Sample was collected 12 hours after intoxication.
In order to set up the analytical method, blood specimens were collected from 80 patients who were admitted to Chungnam National University Hospital from February to July in 2015. The collected specimens were respectively stored in EDTA solution at 4 ℃ until analysis. GCS score, mental change, elapsed time from intoxication and estimated dose of patients were recorded at the time of admission.
Blank human whole blood was purchased from the Korean Red Cross (Daejeon, Korea) and used for validation of the analytical procedure. Zolpidem standard and trimipramine-d3, taken as the internal standard (IS), were purchased from Cerilliant (Round Rock, TX, USA). All solvents were products from Burdick & Jackson (Muskegon, MI, USA), of HPLC grade.
Stock solution of zolpidem was prepared at 1 and 10 μ g/mL in methanol, and trimipramine-d3 was prepared at 5 μ g/mL. Working solutions were prepared by diluting stock solution to appropriate concentrations. All solutions were stored at 4 ℃.
Zolpidem metabolite, collected from patients in hospital.
Solid-Phase Extraction (SPE) cartridges (Bond Elut Certify, 130 mg/3 mL, Agilent Technologies, Santa Clara, CA, USA) were used for sample preparation.
3.3.1 Sample preparation for screening of patients samples
For each sample, a 1.0 mL aliquot of blood sample and 3 mL of 0.1 mol/L phosphate buffer (pH 6.0) were added into a test tube. The mixture then followed to undergo: 2 minutes of vortex, 10 minutes of sonification and 10 minutes of centrifugation at 3500 rpm. 3 mL supernatant of the mixture was loaded into an SPE cartridge preconditioned with 2 mL methanol and 2 mL of 0.1 mol/L phosphate buffer. The cartridge was then washed with 1 mL water and 1 mL 0.2 mol/L acetic acid. Vacuum (15-in Hg) was applied for 4 minutes to dry. 50 μ L of methanol was applied, and then dried for 1 minute (vacuum, 15-in Hg).
The acidic analytes were eluted with 3 mL of chloroform/acetone (1:1, v/v) (Acidic fraction). The basic analytes were eluted with 3 mL of 2% ammoniated ethyl acetate (Basic fraction). The eluted substances were evaporated using nitrogen gas (50 ℃). The extract was reconstituted with 100 μ L of methanol, from which 1 μ L was injected into the GC-MS for analysis.
3.3.2 Sample preparation for quantification of zolpidem
A 1.0 mL aliquot of blood sample was added into a test tube, together with 30 μ L of IS (trimipramine-d3) and 3 mL of 0.1 mol/L phosphate buffer (pH 6.0). The following steps were same as those for patients samples in 3.3.1 above, with the exception of second dryness at vacuum of 1-in Hg.
3.4.1 GC-MS condition for screening of patients samples
An Agilent 7890B GC system coupled with an Agilent 5977A MSD (Agilent Technologies, Santa Clara, CA, USA) was used in an assemblage of the HP-5MS fused silica capillary column (30 m × 0.25 mm i.d., 0.25 μ m film thickness, Agilent Technologies). Splitless mode was selected. Helium was chosen as the carrier gas at the flow rate of 1.0 mL/min. The oven temperature was set at 80℃ initially for 1 min, and then programmed to increase to 300 ℃ by 20 ℃/min to hold for another 15 min. The temperatures at injection port and transfer line were 300 ℃ and 250 ℃, respectively. The mass spectrometer was operated at 70 eV on electron impact mode. Analysis was performed in scan mode (m/z 45 ~ 550).
3.4.2 GC-MS condition for quantification of zolpidem
Identical apparatus was applied as in 3.4.1. Split mode (20꞉1) was selected. The carrier gas and running were same as those in 3.4.1. The oven temperature was set at 120° C initially for 1 minute, and then programmed to increase to 300° C by 30° C/min to hold for another 3 min. Following the same temperature setup at injection port and transfer line as in 3.4.1 plus the mass spectrometer operating, the analysis was performed in selected ion monitoring (SIM) mode, with the parameters of m/z 235, 307 and 219 for zolpidem and m/z 61, 249 and 297 for trimipramine-d3.
The method for quantification was validated by measuring linearity, extraction efficiency, limit of detection (LOD), limit of quantification (LOQ), precision, and accuracy [13].
Calibration curve was constructed for quantification by injecting 6 different concentrations (20, 50, 100, 200, 500 and 1000 ng/mL) of zolpidem into blank specimens. Extraction efficiency was estimated by comparing the injected solution with the extracted one. LOD and LOQ were determined through signal-to-noise (S/N) at 3:1 and 10:1. Precision and accuracy were tested by replicate analyses (n=3) of repeating 3 times for 3 days. Precision was calculated by the percentage of either relative standard deviation or accuracy.
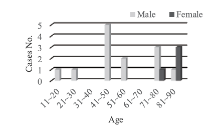
Patients admitted to the emergency room of Chungnam National University Hospital from February to July in 2015 were examined. 80 patients (46 males and 34 females) were intoxicated, and among them, 15 patients (18.7%, 12 males and 3 females) were intoxicated with zolpidem (Fig. 1). The ages ranged from 20 to 88, with the average of 60.
Through analysis of 80 patient`s samples from Chungnam National University Hospital, zolpidem was the most frequent drug, identified in 15 cases, followed by 9 cases of diphenhydramine, 8 cases of tramadol and acetaminophen, 6 cases of chlorpheniramine, 5 cases of quetiapine, and 4 cases of doxylamine. Imipramine, temazepam and trazodone were of 3, respectively. Diazepam, phenobarbital, dihydrocodeine and carbamazepine were of 2, individually.
Most of the drugs such as benzodiazepine were analyzed through basic fraction, but acetaminophen and phenobarbital were analyzed in acid fraction.
The method was validated before analyzing zolpidem from actual samples. Linearity, LOD and LOQ, extraction efficiency, and precision and accuracy of zolpidem out from GC-MS were the tested parameters.
The calibration curve was linear over the concentration range of 20 - 1000 ng/mL, with the correlation coefficient of r2 =0.9997 (Fig. 2). The extraction efficiency ranged from 64.4% to 77.7%. LOD and LOQ values were 1 and 10 ng/mL, respectively (Table 1). The intra- and inter-day precision and accuracy were acceptable, ranging from 0.4% - 1.85% and 86.82% - 113.72%, respectively (Table 2).
| Table 1 Method validation for zolpidem |
| Table 2 Intra- and inter-day precision and accuracy |
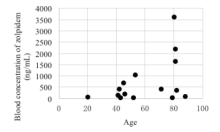
The blood concentration of zolpidem in 15 patients ranged from 19.63 - 3605.85 ng/mL (Table 3). In cases of ingesting zolpidem alone (n=5), the blood concentration was spanning from 20.04 - 3605.85 ng/mL, while bestriding across 19.63 - 2195.85 ng/mL in the cases involving multiple drugs (n=10). According to Schulz [12], the blood concentration of zolpidem amid 80 - 200 ng/mL is therapeutic concentration, toxic for 120 - 700 ng/mL, and comatose-fatal across 1600 - 7700 ng/mL. Therefore, 3 cases were classified as comatose-fatal, 4 as toxic, 2 as therapeutic and 5 as less than therapeutic in this study. In terms of fatal concentration of zolpidem, it has been reported that the blood concentration of the zolpidem spiked with alcohol was 800 - 900 ng/mL, while in two cases resulting in death from zolpidem ingestion alone, the blood concentrations were even 4300 and 7900 ng/mL [14]. In addition, according to Chung [11], blood concentration of zolpidem was 100 - 2620 ng/mL in 16 deaths. In Jones [8], the average blood concentration was 300 ng/mL in deaths related to zolpidem. Jonsson [9] reported that the average blood concentration was 1500 ng/mL in deaths by zolpidem alone. Tominaga [10] discovered that the blood concentration of zolpidem ranged from 60 - 240 ng/mL in 3 deaths. In other studies [15, 16, 17, 18], 520 - 5000 ng/mL of zolpidem was detected in post-mortem cases. Thus, fatal concentration of zolpidem differs significantly by case from 60 to 7900 ng/mL. These results indicate that various factors such as dose, elapsed time after intoxication and co-administration with other drugs have their own and/or combinatorial effect. Therefore, toxicokinetics studies on zolpidem should be carried out.
Zolpidem is absorbed readily from the gastrointestinal tract. First-pass hepatic metabolism results in oral bioavailability of 67%, and 92% of the absorbed drug binds with plasma proteins. Zolpidem has a half-life of 2.2 + 0.4 hours, but shorter in children (less than 1.4 hours) and longer for elders (about 2.8 hours). Patients with hepatic cirrhosis show longer half-life of about 9.9 hours [19, 20]. After single oral administration of 10 mg, the peak blood concentration was 120 ng/mL in average at 1.6 hours, and 230 ng/ml on 2.1 hours for 20 mg [21, 22, 23, 24, 25, 26, 27]. In the case of overdosing 300 mg of zolpidem, added with 600 mg of prothipendyl (a neuroleptic) and/or ethanol, the zolpidem blood concentrations at 3, 4, and 5 hours after ingestion were 550, 435, and 290 ng/mL, respectively [28].
Out of the 15 patients, 5 (33%) had ingested zolpidem for suicide attempt; 2 patients suffered from depression; schizophrenia, anxiety disorders and chronic alcoholism had 1 case for each. There was 1 patient suffering from Alzheimer’ s and Parkinson’ s diseases. The elapsed time after intoxication varied, ranging from 1.5 to 25 hours until analysis, which was carried out within 12 hours for 9 cases and 24 hours for 14 cases. The longest time elapsed was 25 hours.
In this study, 7 were elderly patients (over 65 years old). The average concentration of zolpidem in the 5 patients of the 80s (year-old) was 1571.81 ng/mL, 2 patients in the 70s was 214.73 ng/mL. And 2 patients in the 50s was 523.79 ng/mL, 5 patients in the 40s was 291.17 ng/mL, indicating the highest zolpidem concentration appearing in the highest age group (Fig. 3). The average concentration of patients over 65 years old was 1184.08 ng/mL (21.64 - 3605.84 ng/mL), and 317.96 ng/mL (19.63 - 1027.54 ng/mL) in patients under 65 years old.
| Table 3 Clinical status and blood concentrations |
In reviewing the GCS score at the time of admission, it ranged from 3 to 15. Semi-coma was observed at GCS 3, stupor at GCS 6, and deep drowsy at GCS 7. Drowsy, stupor and confuse were seen at GCS 8 and 9. Alert and drowsy were both observed at GCS 12 and 15. In comparing GCS score with blood concentration of zolpidem, the two measurements did not agree. The average concentration of zolpidem was 149.84 ng/mL at GCS 15, 1327.65 ng/mL at GCS 14, 129.51 ng/mL at GCS 12, 414.25 ng/mL at GCS 10, 139.04 ng/mL at GCS 9, 1444.2 ng/mL at GCS 8, 199.9 ng/mL at GCS 7, 3605.85 ng/mL at GCS 6, and 72.42 ng/mL at GCS 3. Additionally, mental change did not agreed with the blood concentration of zolpidem. Thus, comparing the clinical symptoms in accordance with the blood concentration is inadequate. Various factors must be taken into consideration in order to determine intoxication patterns of zolpidem.
The blood concentration of the patient who allegedly ingested 500 mg of zolpidem was 414.5 ng/mL, whereas that of the patient who allegedly ingested 50 mg was 3605.85 ng/mL. There was no correlation between estimated dose and blood concentration of zolpidem. Even in the cases of 8 and 13, the administration of 250 mg resulted in different concentrations of 1627.77 and 692.55 ng/mL. Also, two cases of 187.5 mg ingestion caused significant difference of 20.04 and 2195.85 ng/mL. In comparing blood concentration with the elapsed time after intoxication, 692.55 ng/mL after 1 hour, 407.83 ng/mL after 1.5 hours and 3605.85 ng/mL after 25 hours were observed. The blood concentration was roughly but not exactly in proportion getting higher when the elapsed time elongated, indicating lack of accuracy on information regarding estimated dose.
Alcohol test was conducted on patients who ingested zolpidem, with 3 patients being tested positive. The blood concentration of alcohol ranged from 0.104 to 0.112% (Table 3). In the case of alcohol level of 0.104%, the blood concentration of zolpidem was low, his mental state was alert and no effects shown of alcohol. For the two cases with the alcohol concentration of 0.106 and 0.112% was showed the mental state of drowsiness, indicating no significant interaction between alcohol and zolpidem. For cases of ingesting zolpidem with alcohol, some alcohol interaction was observed with the ingestion of 10 mg of zolpidem, while very little interaction was observed with the ingestion of 15 mg or greater, coinciding with previous studies [21, 29, 30, 31].
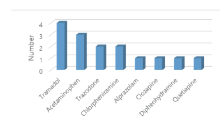
Among patients intoxicated with zolpidem, 10 cases involved ingestion of other drugs. The most frequently taken concurrently was tramadol, identified in 4 cases, followed by acetaminophen of 3, and trazodone and chlorpheniramine of 2, respectively. Alprazolam, clozapine, diphenhydramine and quetiapine had 1 case reported for each (Fig. 4).
In cases where zolpidem was ingested alone, the mental change was alert at blood concentration of 20.04 ng/mL and drowsy around 407.83 - 414.25 ng/mL. Despite some consistency in the observed patterns, mental change of stupor is also observed at 357.18 and 3605.85 ng/mL, showing no correlation between blood concentration and mental change. Establishing correlation between the two components was even more difficult in cases of multiple drug ingestion.
In order to correlate the blood levels with clinical symptoms, it is a must to develop one method to define GCS score and mental change along with obtaining the accurate information of amount ingested.
The concentrations of zolpidem in certain patients from, e.g., Cases 6, 8 and 9, revealed 3605, 1627 and 2195 ng/mg, which could be comatose-fatal according to Schulz [12]. However, mental changes of the three involved patients were stupor, alert and drowsy when admitted, demonstrating not serious with comparison to the zolpidem level in blood. This result is compatible with the report by Suh et al. [32]. They reported that the clinical course couldn’ t be predicted by zolpidem concentration, concluding that the acute zolpidem intoxication is mild in progress. They determined the blood concentration of zolpidem in 30 patients ranging from 200 to 7400 ng/mL by REMEDi approach, observing that all patients recovered to full consciousness. According to Garnier [33], 4 cases of coma plus only 1 case of respiratory trouble were observed out of 344 cases. In Wyss [34], only 1 case of coma was observed out of 54 cases. These studies indicate relatively moderate intoxication symptoms and easy recovery from zolpidem. Even the patients, who showed fatal concentration of zolpidem, recovered and were discharged within a few days. As Garnier stated [33], major intoxication symptom of zolpidem is decreasing in consciousness with moderate clinical outcomes.
We developed a fast and accurate analytical method for screening and quantifying drugs from acute intoxication patients using GC-MS with SPE. As a result of analyzing 80 patient-samples from Chungnam National University Hospital, zolpidem was the most frequently detected drug, identified in 15 cases. Method validation for zolpidem was carried out. The zolpidem analysis method showed a good linearity, low LOD and LOQ, as well as the appropriate extraction efficiency, accuracy and precision. From 15 zolpidem intoxication patients, blood concentration was measured and compared with clinical symptoms.
Out of 15 intoxication patients, 33% ingested zolpidem for suicide attempt. Ages ranged from 20 to 88. Five cases involved zolpidem ingestion alone, while 10 cases had co-administration of other drugs, and 3 cases were of alcohol added. In terms of drugs taken together, tramadol was detected in 4 cases, acetaminophen in 3, and trazodone and chlorpheniramine in 2, respectively. Alprazolam, clozapine, diphenhydramine and quetiapine were each identified in 1 case.
The blood concentration of zolpidem in 15 patients ranged from 9.63 - 3605.85 ng/mL. Five patients in their 80s showed the highest concentration. However, no correlation was established between blood concentration and GCS score or mental change, as well as the amount ingested, and the elapsed time after intoxication. Since patients in the older age group showed higher blood concentration, individual difference in metabolism seems to have a greater effect than dose.
Even, patients who showed high blood concentration of zolpidem improved within a few days and were discharged. Therefore, intoxication from zolpidem overdose is relatively moderate, not leading to further damage.
This study will supplement analytical system for detecting drugs more quickly from emergency patients, information of correct dose, and toxicokinetics researches to establish a proceeding for fast and appropriate treatment. In addition, it will provide useful information for determining intoxication deaths related to zolpidem in forensic toxicology.
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (MSIP) (2018, R& D Equipment Engineer Education Program, 2014R1A6A9064166) and a grant (16182MFDS382) by Ministry of Food and Drug Safety, Korea in 2018.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|