作者简介: 秦 达(1983—),男,吉林省吉林市人,助理研究员,博士,研究方向为文件检验. E-mail: qinda@cifs.gov.cn
烧毁文件是指因人为故意或过失,或者因火灾事故,使具有物证,书证作用的文件被燃烧后的残留物.文件烧毁后,纸张变脆,保存能力差,通常呈现焦化,炭化或灰化三种表观变化状态,但是这三种状态的形成机理及其如何区别一直没有一个清晰的界定,导致三个词语的混淆使用.本文采用热重分析方法对烧毁文件进行分析,研究纸张烧毁的不同程度和阶段,并对其烧毁机理进行探讨.首先采用热重法(thermogravimetric analysis, TGA)对5种纸张进行测试,得到了空气和氮气气氛下的不同热重曲线,结果表明:(1)5种纸张的热重曲线形状基本相同,均存在着三个明显的失重过程,说明纸张随温度升高的变化机理相同;(2)其他参数不变,随着升温速率的增大,热重曲线向高温区方向移动.尽管纸张的起始分解温度及最大热分解速率温度有所差异,但是热重曲线的形状基本保持不变;(3)复印纸的热重(thermogravimetric, TG)曲线和微商热重(derivative thermogravimetric,DTG)曲线表明,氧气的存在可以加速纤维素的分解.氧气浓度增加,DTG曲线上纤维素以及残留的焦的分解峰向低温方向移动,峰形变化也比较明显;(4)室温状态下逐渐升温,纸张质量降低,至120°C时到达一个平台,该部分失重为纸张中纤维素物理吸附的水分,此过程中纸张的性质基本没有发生变化.从210°C到470°C,纸张有较多的失重,主要是纸张中纤维素分解造成的.从580°C到670°C,失重是由于高温下填料(主要是碳酸钙)的分解造成的.温度继续增加,质量变化不大,残余物为纸张中热稳定的物质,为纤维素氧化后的炭和填料等.在210°C到470°C范围内,纤维素开始时并没有完全分解,部分裂解为焦;345°C后残留的焦氧化为炭和挥发性气体,在图像上显示为两部分斜率不同的曲线;(5)牛皮纸由于其纤维素木浆成分较多,升温时极易剧烈氧化并燃烧,在空气气氛中DTG曲线有一个尖锐峰,与其他纸张有所差异,氮气气氛中差异不大.在纸张升温过程中,存在纤维素的降解,裂解两个竞争过程,造成了纸张在不同温度下发生物理和化学变化.从热重研究和表观状态研究与纸张燃烧时的化学反应状态并结合具体燃烧机理,可以得出文件纸张烧毁的四个阶段:(1)失水阶段:温度在100°C以下,纸张变得干燥,颜色略微发黄.主要是该温度范围下纸张中纤维素物理吸附的水分蒸发,纸张的基本物理性质没有太大变化;(2)焦化阶段:燃烧温度为150°C~200°C,纸张干燥,颜色开始变黄,起皱,边缘翘起和卷曲;燃烧温度200°C~250°C,纸张呈深褐色转黑色,面积缩小,卷曲加重.从机理上看是纤维素开始分解,但是由于燃烧环境中气氛的不同,纤维素并没有完全分解,部分裂解为焦.此阶段为纤维素脱水和裂解的竞争阶段;(3)炭化阶段:燃烧温度250 °C~300°C,纸张逐渐完全燃烧,炭化并断裂;燃烧温度300°C~350°C,纸张开始灰化,并呈灰黑色.从机理上看,随着温度的进一步升高,裂解为小分子量的焦继续氧化,生成CO2,H2O和CO,剩余的固体为炭化的纤维素;(4)灰化阶段:燃烧温度350°C ~400°C以上,纸张烧成灰白色,几乎成粉末状灰烬,从机理上看,是上一个阶段残留的炭继续氧化,剩余固体为纸张中残留的不易氧化的填料等.此外,在该阶段填料中碳酸钙也会分解,造成残留质量的进一步减少.该阶段所处的状态为灰化.对应热重曲线最后的部分.除了在灰化阶段,纤维素基本都被氧化分解,填料中碳酸钙等物质也在高温中分解,整复难度很大外,在纸张烧毁的前三个阶段,即脱水,焦化,炭化阶段,只要通过适当手段熄燃,纸张中还残留有起到支撑作用的高分子纤维素,纸灰还可以整复.因此,准确判断纸张烧毁的不同阶段,可以为烧毁文件的检验奠定整复和辩读的基础.
The examination of charred document is a challenge and usually requires a careful application of certain scientific techniques due to its unstable property. To address this issue, the mechanism of paper burning was studied in this paper. Here thermal-gravimetry (TG) was applied to investigate five kinds of paper, along with their TG and derivative thermogravimetric curve (DTG) observed at different atmospheric conditions. The results showed that the shape of curves, albeit similar, varied with the physical and chemical composition of paper. In the burning process, dehydration and de-polymerization are the two main pathways for cellulose, the major ingredient of paper. The heating rate indicated little influence on the curves while the sort of atmosphere worked strongly. The reason is due to the lack of tar oxidation when nitrogen used as the atmospheric environment. At moderate temperature, de-polymerization prevails and the tar can be observed. With temperature increasing, the tar and cellulose are further decomposed, leading to products of high boiling-point. According to the results, the charred document can be classified as one of the dehydrated, tarred, charred and ashed. Except the ashed stage, the other three can be handled and the writing whereon can be deciphered. The results exposed hereof may provide a fundamental for examining and deciphering charred document.
In relevant criminal cases, burning is often used as means to destroy documentary and/or material evidence to mask criminal facts. There are also some burned files or documents left in the scenes of explosion or fire. The examination of burned documents is helpful to find out the cause of the accident and/or detect the criminals[1, 2]. For document examination, charred document usually refers to the damaged paper by intentional or unintentional action to make it into state of the charred, carbonized or ash [3]. However, at present the difference among the three states, charred, carbonized and ash, is neither clearly understood nor did the mechanism of the charring process. These three words are often confused and thus compromise the examination of charred document. To clarify the mechanism of burned document can provide the scientific definition to distinguish the exact state of burned document. Thermal analysis (TA) is a branch of materials science where the properties of materials are studied on changing with temperature. TA is widely used in forensic science, like examining the synthetic fiber or shotgun slug [4, 5]. As one of TA, thermogravimetric analysis (TGA), now popular, measures the changes in physical and chemical properties of materials as a function of temperature (increasing at constant heating rate) or time (under constant temperature and/or constant mass loss), thus providing the information about physical and chemical phenomena. According to thermogravimetric (TG) curves, derivative thermogravimetric (DTG) curve can be obtained to give more information about the material. Here, TG and DTG curves were used to investigate the mechanism of paper burning in combination with the analysis of its apparent change.
Duplicating paper, newspaper, account book laid azure, kraft paper, and Xuan paper were selected as the test stuff (Table 1). About 5 mg of the sample was placed in the crucible of Q50 Thermal Gravimetric Analyzer with its heating temperature set from room temperature to 900° C and a 10° C/min of the heating rate that was fixed upon testing different rate of temperature increasing. The atmospheric environment was air or nitrogen (N2) and α -Al2O3 as reference.
Muffle furnace (Model SX2-B-13DZ, Shanghai Pudong Rongfeng Scientific Instrument Company, Ltd) was used to study the paper’ s apparent change with the temperature increasing from room temperature to 550° C. The sample’ s apparent state was recorded at the interval of every 50° C elevated.
| Table 1 Paper selected for test |
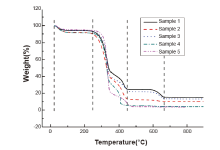
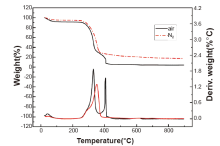
TG and DTG curves of 5 kinds of paper were obtained with air flow under the heating rate of 10° C/min (Fig.1). The results showed that the shape of curves is similar: as temperature gradually increases from room temperature, the paper’ s mass is decreasing and has reached a platform at about 120° C. From 210° C to 470° C, the paper has lost much of its weight; and from 580° C to 670° C, the weight, after continuing to decrease for some duration of time, keeps nearly constant even if the temperature still increasing. In the range of 210° C to 470° C, there are two descending segments of different slopes in each of the curves, with sample 4 (kraft paper) the sharpest. As shown in Fig.1, the shape of curves under air atmosphere is similar but varies due to different physical and chemical composition of paper.
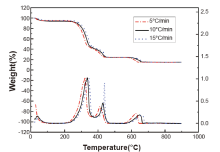
Heating rate is an important factor in TG technique. To investigate the influence to TG curves, three heating rates of 5, 10 and 15° C/min were applied in TG of a typical electrostatic duplicating paper. Shown as Fig.2, TG curves move to the high temperature area with the heating rate increasing. That is probably because of the higher heating rate, the shorter heating time and the lower reaction degree for samples to reach the same temperature. Also, the temperature gradient and heat-transfer coefficient are affected by heating rate, resulting in a slow-down response for the instrument to follow the real track of paper losing weight, thus making TG and DTG curves shift to the high temperature zone. However, although the change of heating rate transforms the initial decomposition temperature and the maximum thermal decomposition rate of the paper into difference, the shape of TG and DTG curves basically maintains unchanged. With the given temperature at three heating rates of 5, 10 and 15° C/min, the information obtained from curves of the same sample was almost the same. Therefore, the heating rate of 10° C/min was fixed in the following experiments in order to guarantee the accuracy of the experiment.
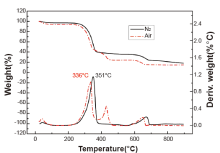
In actual cases, the consequence and burned degree of documents have closely related to oxygen content. For example, the burned degree is quite different on paper being lit in ordinary atmosphere from that in a violent explosion. In order to eliminate the influence of tested atmosphere on TG curve of paper and simulate the low oxygen environment, the tests of 5 kinds of paper under nitrogen and air atmosphere were conducted. The results of different paper were similar, and typical curves of electrostatic duplicating paper were investigated, as shown in Fig.3.
In nitrogen atmospheric condition, the peak at 351° C on DTG curve can be attributed to decomposition of the cellulose of paper but the one sitting at 600° C ~700° C to the decomposition of calcium carbonate (CaCO3). In air atmosphere, these two peaks also exist, indicating that paper underwent similar process during burning. In the oxidative atmosphere (air), one more peak appears in the DTG curve where there are two corresponding decreases in the TG curve, a result deduced to the oxidation of the residual tar being born from cellulose decomposition. From the results, we can see that the presence of oxygen can accelerate the decomposition of cellulose. The decomposition peaks of cellulose and tar residuals shift to lower temperature area with the increasing of oxygen concentration, leading to an obviously shape-changed curve [6].
Generally, paper is composed of plant cellulose, additive, adhesive, pigment and among others, amidst which the cellulose is the major ingredient to keep structure. With temperature increasing, the cellulose is dehydrated and de-polymerized with its polymerization reduced. Eventually most of the cellulose has been oxidized with high boiling-point products left only. The burning of paper is a complex process of chemical reaction which is influenced by many physical and chemical factors including the composition of the paper, combustive atmosphere, crystallization, impurity and among others. In the burning process, dehydration and de-polymerization are two competitive pathways. If the dehydration of cellulose prevails, the evolution of carbon dioxide (CO2), water (H2O) and carbon monoxide (CO) is primarily observed with the formation of solid char. If de-polymerization excels more extensively than dehydration, the tar will be produced [7].
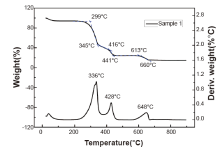
As shown in Fig.4, the mass of the paper declines when temperature increases. At about 120° C, the curve drops to a platform at the weight loss of 6%, a cause of evaporation of the water integrated into cellulose. At this stage, the property of paper has not significantly changed. In the range of 210° C to 470° C, the weight of paper loses about 69.6%, being resulted from the decomposition of cellulose. In the temperature range from 580° C to 670° C, the decomposition of filler materials (mostly CaCO3) brings about the further weight loss around 9.1%. At higher temperature, the mass is almost constant with high boiling products being kept.
In the range from 210° C to 470° C, two parts of descending curves can be observed. The first part indicates that cellulose is not completely decomposed and tar being produced through de-polymerization. From 345° C to 470° C as the second part, the residual tar continues to oxidize, hence the solid char is observed.
Pulp kraft paper is made from softwood by the kraft process. The long fibers provide the paper with both native and wet strength which is even further improved when necessary chemicals added. As shown in Fig.5, the water integrated into cellulose accounts for about 5%~8% of the paper weight and 4% as of for the residual stuff survived after high temperature under nitrogen atmospheric environment. This value shows that the softwood pulp is the main component in this kind of paper. In the air atmosphere, there is a sharp-pointed peak at 406° C in DTG curve, revealing the intense burning occurring. The phenomenon did not appear in the other tests. The reason may be due to high proportion of the wood pulp in kraft paper, which is easily burned in the experiment [8].
When paper is burned, certain physical and chemical reactions take place in succession of water evaporation, shrinking, wrinkling, color changing and carbonization at apparent state. Here, the experiment of apparent change of paper was conducted as shown in Fig.6. When temperature increasing to 100° C, paper was dry and its color turned yellowish, and further became yellow and curled edges when 150~200° C climbing, and consecutively changed from dark brown to black together with being much curlier and the dimension turning smaller at 200~250° C stepwise elevating [9], and finally converted from grey white into ash as 350~400° C ascending.
From TG and DTG and observation of the apparent state, it can be concluded that the basic process of physical and chemical reaction is similar for different kinds of paper. According to the results, four stages of paper burning can be put forward: (1) Dehydrated: Below 100° C, paper is slightly yellow. In this stage, water absorbed in cellulose is gradually lost, and the basic physical property keeps intact. (2) Tarred: From 150° C to 250° C, paper color changes from yellow to brown, and the edges becoming curly along with the dimension turning small [10]. In this stage, though cellulose is not completely decomposed, yet tar is obtained through de-polymerization. (3) Charred: From 250° C to 350° C, paper changes from dark brown to black and becomes much curlier. In this stage, residual tar continues to oxidize, thus solid char being observed. (4) Ashed: Above 350° C, paper turns from grey white to ash. In this stage, char continues to oxidize, leaving the high boiling-point products, mainly the filler materials (mostly CaCO3). In the stages of dehydrated, tarred and charred, the charred document can be handled because of cellulose still existing in paper. However, the ashed document is difficult and even impossible to handle as a result of complete decomposition of the cellulose.
In this paper, thermal-gravimetry technique was applied to investigate the mechanism of paper burning in combination with the apparent change analysis. The more accurate definition of four stages of burned documents will be beneficial for questioned document examiners to better understand the burning state of charred document.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|